The role of glucose in metabolic health
Glucose is far more than just the body’s primary energy source—it also plays a critical role in regulating gut function and overall metabolic health. Beyond its systemic effects on blood sugar levels, recent research highlights glucose’s direct impact on glucose metabolism, gut motility, and its interaction with the enteric nervous system (ENS). This complex interplay has significant implications for conditions like type 2 diabetes, where disruptions in glucose regulation contribute to metabolic disorders.
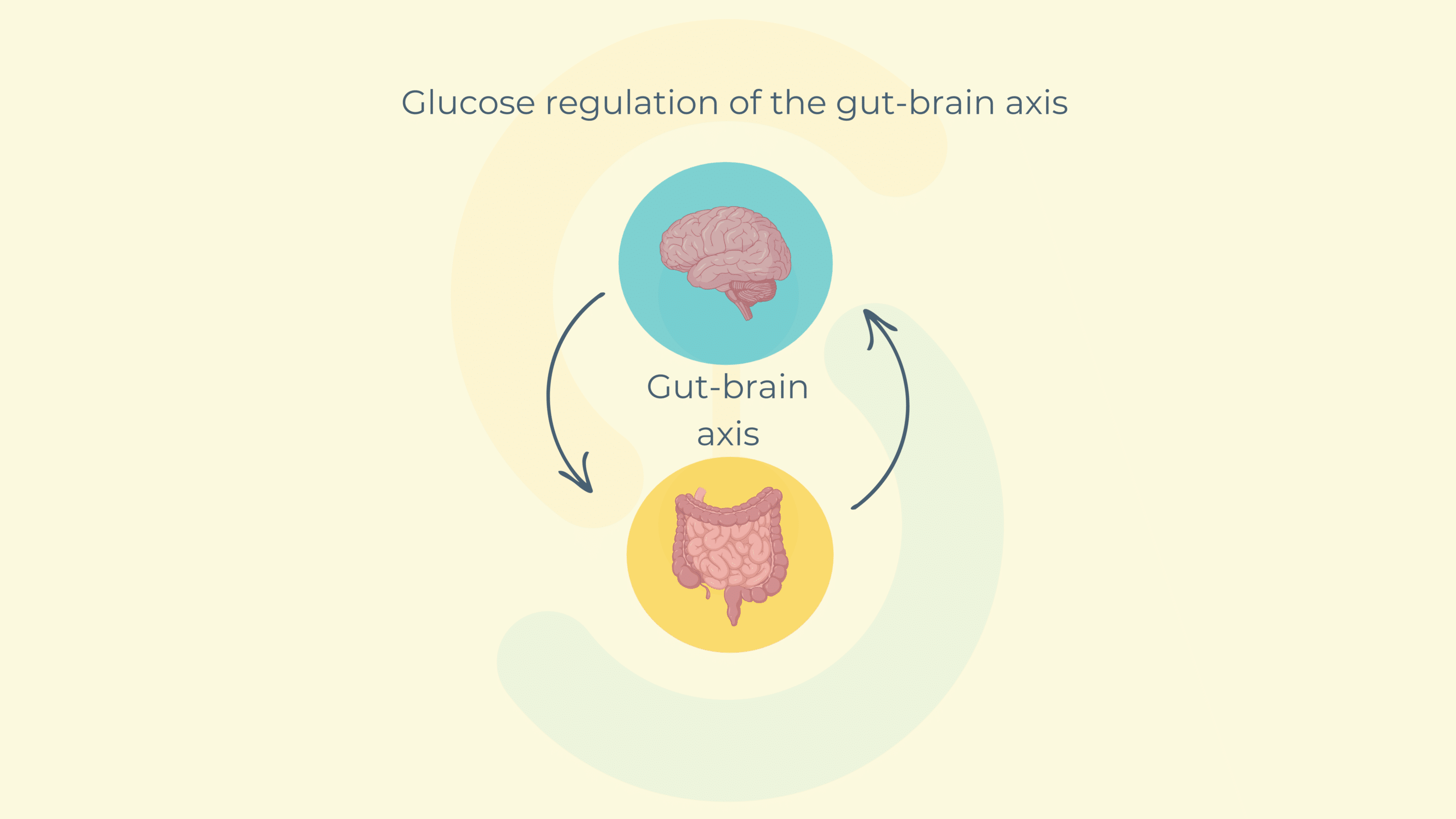
At the center of this connection lies the gut-brain axis, a bidirectional communication pathway that connects gut activity with brain signaling. When glucose is detected in the small intestine, mechanoreceptors and chemoreceptors send neural signals to the brain’s hypothalamus, which, in turn, regulates glycemia and peripheral glucose uptake in tissues such as the liver, skeletal muscle, and adipose tissue .
However, in pathological states like type 2 diabetes, this communication becomes disrupted. The intestinal motility becomes hyperactive, glucose sensing is impaired, and hypothalamic signaling is altered. Understanding how glucose impacts gut motility—and how this can be modulated—could unlock innovative therapeutic strategies for better glucose regulation and metabolic health.
Exploring glucose, gut motility, and enteric nervous system activity
In a recent study, Enterosys, a leader in preclinical research on gut-organ interactions, investigated the role of this enterosyne in gut motility under both fasted and fed conditions in diabetic and non-diabetic mice models.
The team analyzed transporter expression levels, specifically GLUT2 and SGLT1, in the duodenum and jejunum of mice fed either a normal chow (NC) or a high-fat diet (HFD). Using ex vivo isotonic contraction sensors, they measured the effects of varying glucose concentrations on gut motility.
To further understand the molecular mechanisms, nitric oxide (NO) release from enteric neurons was measured in vitro, providing insights into how this enterosyne influences gut contractions at a neuronal level. The inhibition of GLUT2 using phloretin allowed researchers to pinpoint the specific transporter involved in glucose-induced changes in gut motility.
The study revealed that glucose plays a direct role in regulating gut contractions. In both fasted and fed states, this molecule significantly increased intestinal motility, with its effects mediated by the GLUT2 transporter. This stimulatory effect was observed in both duodenal and jejunal segments and was most pronounced at glucose concentrations starting from 5.5 mM .
Interestingly, glucose-induced motility was closely linked to a decrease in nitric oxide (NO) release from enteric nervous system neurons. NO is a critical inhibitory neurotransmitter in the ENS, responsible for regulating smooth muscle relaxation. The observed reduction in NO suggests that glucose may act by inhibiting neuronal NO release, leading to increased gut motility.
However, these effects were notably absent in diabetic mice fed a high-fat diet. Despite increased GLUT2 and SGLT1 expression in the gut lining of diabetic mice, glucose failed to modulate gut contractions or NO release effectively. This suggests a breakdown in glucose sensing and signaling pathways within the gut-brain axis during diabetes .
When GLUT2 was pharmacologically inhibited using phloretin, glucose-induced motility was completely blocked, reinforcing GLUT2’s essential role in this process. These findings indicate that glucose transport and ENS signaling are tightly intertwined and collectively influence metabolism and gut motility.
Implications for diabetes management
The study underscores the dual role of this enterosyne as both a metabolic substrate and a regulator of gut motility. By modulating gut contractions through the GLUT2 transporter and neuronal NO pathways, glucose directly influences glucose metabolism and absorption rates in the intestine.
In pathological states such as type 2 diabetes, impaired glucose sensing in the gut leads to hypermotility, increased the absorption, and disrupted hypothalamic signaling. This disruption exacerbates hyperglycemia and insulin resistance, creating a vicious cycle of poor metabolic control .
Targeting transporters like GLUT2 and modulating NO release within the enteric nervous system offer promising therapeutic strategies. Future treatments could involve pharmacological agents or nutraceutical approaches that restore gut-brain communication, improve glucose regulation, and reduce systemic hyperglycemia.
Partner with enterosys for innovative gut-organs research
At Enterosys, we specialize in uncovering the intricate connections between gut health and systemic metabolic processes. Our preclinical research offers valuable insights into metabolism, gut motility, and the role of the enteric nervous system in metabolic disorders.
Collaborate with Enterosys to pioneer innovative solutions for gut-brain axis therapies!
Source :
Wemelle E, Carneiro L, Abot A, Lesage J, Cani PD, Knauf C. Glucose Stimulates Gut Motility in Fasted and Fed Conditions: Potential Involvement of a Nitric Oxide Pathway. Nutrients. 2022 May 23;14(10):2176. doi: 10.3390/nu14102176. PMID: 35631317; PMCID: PMC9143273.