In recent years, dermatological research has increasingly turned inward, toward the gut!
The concept of the gut–skin axis has gained considerable traction, supported by robust data linking intestinal health and microbial balance to the onset and progression of numerous skin disorders. This emerging framework is reshaping how clinicians, researchers, and health professionals approach the diagnosis and management of chronic dermatological issues.
Rather than treating the skin in isolation, a growing body of evidence points to the necessity of assessing gut health in patients with persistent or inflammatory skin conditions.
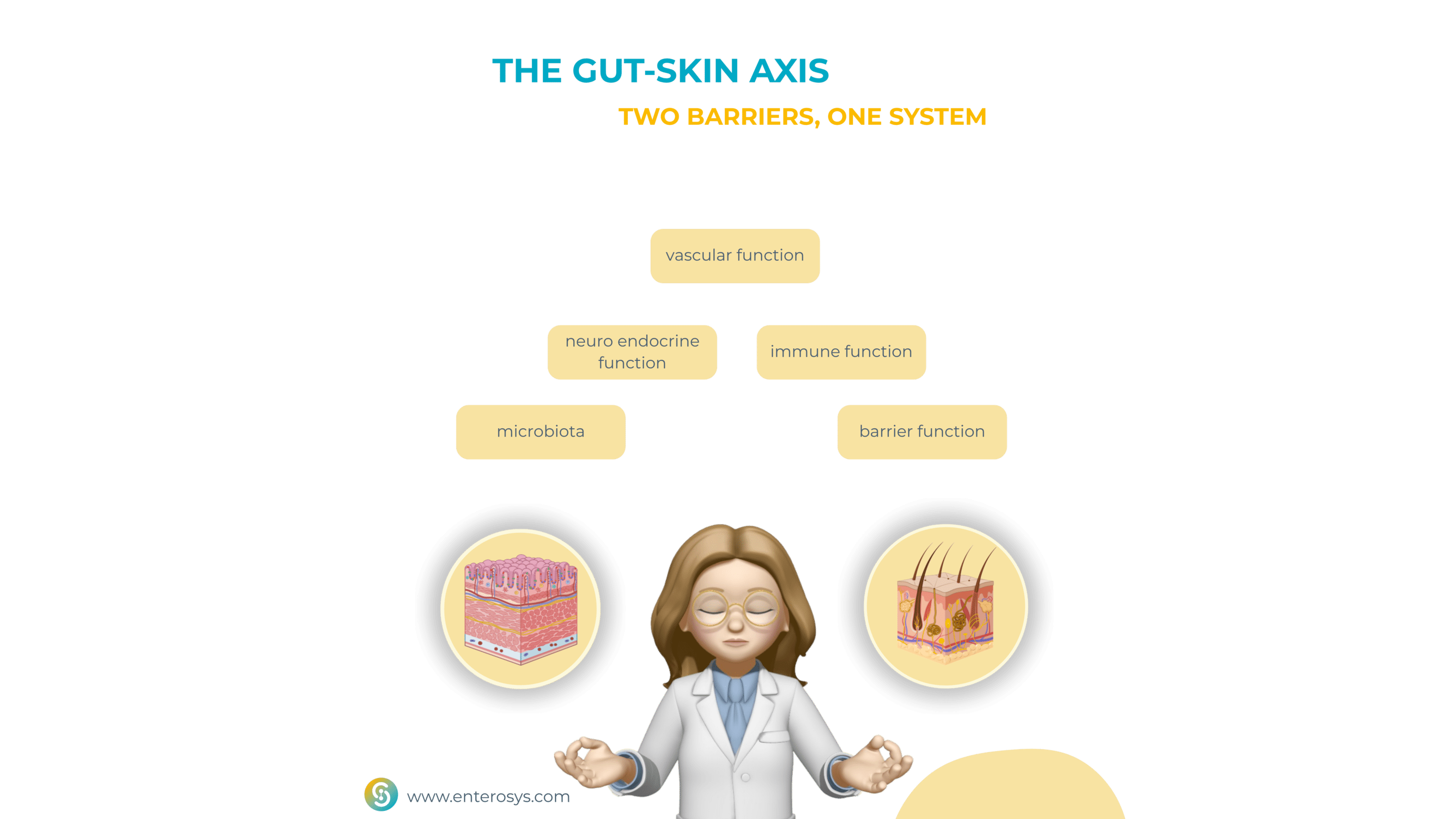
Understanding the Gut–skin axis
The gut–skin axis refers to the bidirectional communication between the gastrointestinal tract and the skin, involving a complex network of immune, metabolic, endocrine, and microbial pathways. Both organs serve as critical immunological barriers, densely populated by microbiota that play key roles in maintaining local and systemic homeostasis.
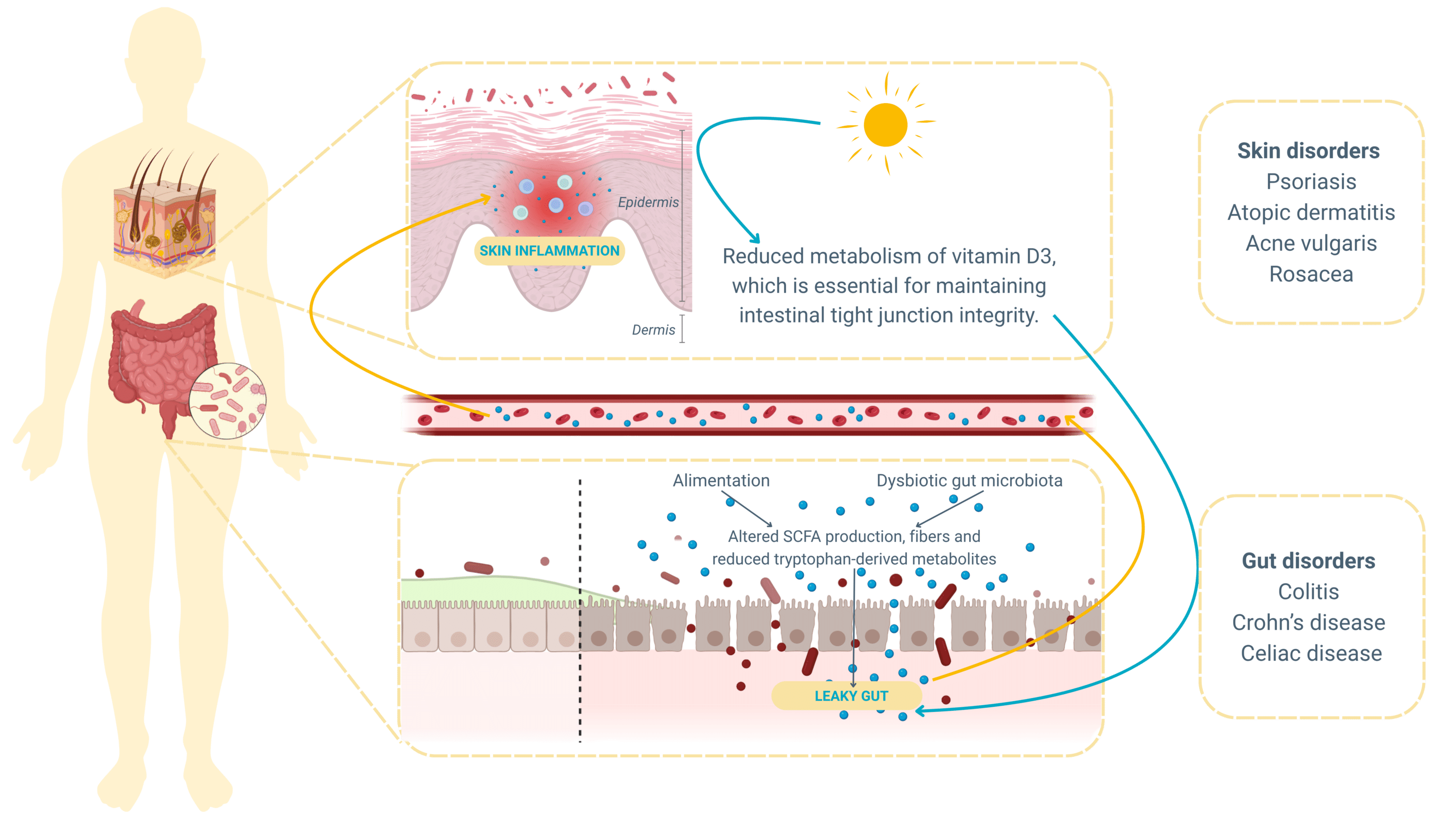
When the intestinal microbiota becomes imbalanced, a state known as dysbiosis, it can lead to increased intestinal permeability, or “leaky gut.” This allows microbial metabolites and pro-inflammatory molecules to translocate into systemic circulation, triggering widespread immune activation that often manifests in the skin.
Curious to learn more? Check out our article on gut microbiota and leaky gut !
Moreover, the gut microbiome produces a vast array of bioactive compounds, including short-chain fatty acids (SCFAs), bile acids, and neurotransmitters. These substances influence skin barrier function, sebum production, immune responses, and even the composition of the skin microbiota itself !
From skin to gut: the reverse axis
While most studies have focused on gut-to-skin signaling, recent findings emphasize a reverse pathway: the skin-gut axis. Environmental stimuli that affect the skin—particularly UVB exposure—can initiate systemic changes that positively influence gut microbiota composition, immune tolerance, and epithelial barrier integrity .
For instance, vitamin D, synthesized in the skin in response to UVB light, activates vitamin D receptors (VDR) in intestinal cells, enhancing the production of antimicrobial peptides like cathelicidin and defensins. These peptides play a crucial role in shaping the gut microbiome and regulating inflammation.
Clinical evidence for the gut-skin axis: linking gut dysbiosis and skin disease
A series of studies over the past decade has established a consistent association between intestinal dysbiosis and various inflammatory skin diseases. The most thoroughly documented include :
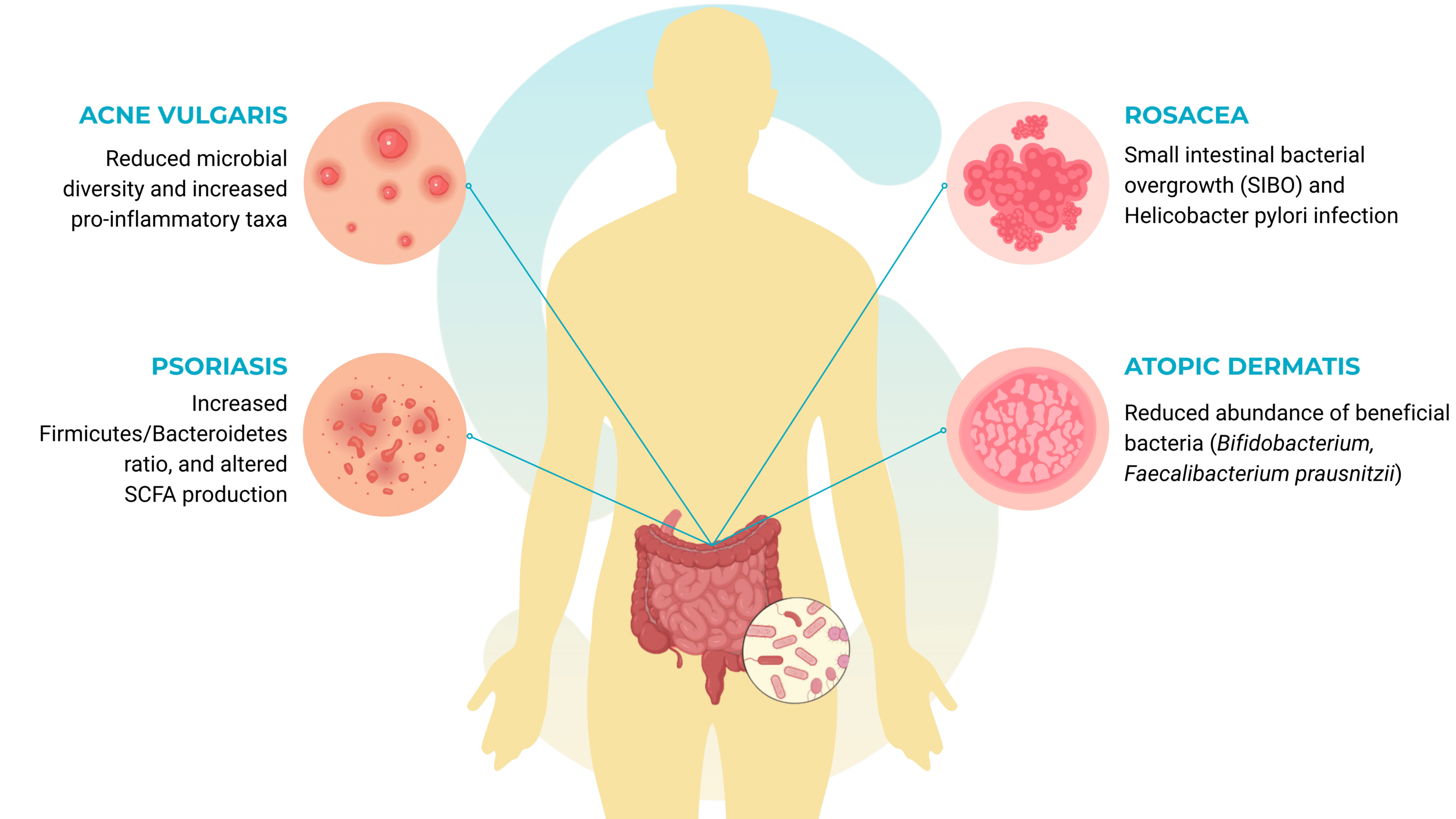
Rosacea
That’s a chronic inflammatory dermatosis, has been linked to gastrointestinal conditions such as small intestinal bacterial overgrowth (SIBO) and Helicobacter pylori infection. Antibiotic treatments targeting these gut conditions have shown significant improvement in skin symptoms, suggesting a causal connection mediated by the gut–skin axis.
Atopic dermatitis (AD)
Psoriasis
Acne vulgaris
Acne vulgaris, often attributed to hormonal or sebaceous dysfunction, also shows signs of microbial involvement beyond the skin. Studies report a distinct gut microbiota composition in acne patients, including reduced microbial diversity and increased pro-inflammatory taxa. Diet-induced dysbiosis further exacerbates this condition by influencing IGF-1 and androgenic activity.
A Paradigm shift in dermatological assessment
Despite the well-established links, current dermatological practice often neglects the intestinal dimension of disease. Yet, if chronic skin conditions share immunological and microbial roots with gut dysfunctions, understanding and addressing the gut environment becomes essential.
Today, advanced gut analysis techniques can assess:
- Microbiota composition and diversity, identifying dysbiotic patterns
- Markers of intestinal permeability (e.g., zonulin, calprotectin)
- Pro-inflammatory microbial metabolites
- SCFA levels and other immunomodulatory byproducts
Such data offer a more integrative view of skin pathology and allow for the identification of upstream drivers that may not be visible through dermatological examination alone.
Implications for therapy: from the gut to the skin
Incorporating the gut–skin axis into clinical decision-making opens new avenues for therapeutic interventions. These include:
- Probiotics, which modulate mucosal immunity, reduce systemic inflammation, and help rebalance both intestinal and skin microbiota. Specific strains such as Lactobacillus reuteri and Bifidobacterium longumhave demonstrated skin-beneficial effects in preclinical and clinical studies.
- Prebiotics and dietary fibers that stimulate the production of anti-inflammatory SCFAs, enhance epithelial integrity, and reduce immune overactivation.
- Targeted dietary modifications, food and feed including anti-inflammatory and low-glycemic-index diets, can influence gut microbial profiles and skin symptoms.
Collectively, these strategies underscore the need for an individualized, gut-informed approach to managing skin diseases that are resistant to conventional treatment or frequently relapsing.
Looking forward: integrating microbiome science into dermatology
The gut–skin axis is no longer a speculative hypothesis but a well-substantiated biological framework that redefines the way we understand and treat gut skin disorders. It challenges the longstanding separation between organ systems and emphasizes the interconnectedness of immune, microbial, and barrier functions.
As microbiome science continues to advance, it is likely that gut-focused diagnostics and therapeutics will become integral components of dermatological care. By recognizing the gut as a key modulator of skin health, we unlock the potential for more effective, lasting, and systemically informed interventions.
The next generation of dermatology will not rely solely on creams and corticosteroids. It will be rooted in systems biology, integrating immunology, microbiome research, and metabolomics to address disease at its source.
The gut is not just a digestive organ—it is an immunological command center. Its signals are written on the skin!
Reevaluating skin symptoms through the lens of intestinal function offers a powerful and scientifically grounded path toward better patient outcomes !
To support and structure this article, the following peer-reviewed scientific publications were used as primary sources of information:
- Lee K, Kim HJ, Kim SA, Park S-D, Shim J-J, Lee J-L. Exopolysaccharide from Lactobacillus plantarumHY7714 protects against skin aging through skin–gut axis communication. Molecules. 2021;26(6):1651. doi:10.3390/molecules26061651
- Salem I, Ramser A, Isham N, Ghannoum M. The gut microbiome as a major regulator of the gut-skin axis. Front Microbiol. 2018;9:1459. doi:10.3389/fmicb.2018.01459
- Szántó M, Dózsa A, Antal D, Kemény L. Probiotics have beneficial effects in atopic dermatitis, psoriasis, acne and rosacea: A review of the current evidence. Exp Dermatol. 2019;28(5):618–627. doi:10.1111/exd.13935
- Jimenez-Sanchez M, Celiberto LS, Yang H, Sham HP, Vallance BA. The gut-skin axis: a bi-directional, microbiota-driven relationship with therapeutic potential. Gut Microbes. 2025;17(1):2473524. doi:10.1080/19490976.2025.2473524