Quick recap : what we covered last time
In my first newsletter we introduced the gut–skin axis: a two-way biochemical hotline in which intestinal dysbiosis, “leaky-gut” endotoxins and microbially produced metabolites ignite cytokine storms that thin the epidermal barrier, while UV-driven vitamin D and cutaneous antimicrobial peptides modulate the gut in return. That primer set the stage; today we drill into the numbers, the newest lab models and the nutricosmetic opportunities.
75 % overlap: when gut disorders meet the dermatologist
Pooled epidemiology now shows that roughly three in four patients diagnosed with a chronic gastrointestinal condition will also receive a skin disorder label during their lifetime.
- A Danish cohort of 4.36 million people revealed that rosacea raises the hazard ratio for coeliac disease (1.46), Crohn’s (1.45) and ulcerative colitis (1.19), alongside irritable-bowel syndrome (1.34).
- In hidradenitis suppurativa, a 2016 meta-analysis pinned inflammatory-bowel-disease comorbidity at 12.8 %, four times the background risk in northern Europe
- Psoriasis follows suit: a meta-analysis covering 7.8 million subjects confirms significant links to Crohn’s and ulcerative colitis and flags a colorectal-cancer signal, especially in women. These population-scale datasets push the gut–skin axis from intriguing hypothesis to clinical reality.
Mechanistic deep dive: from leaky gut to leaky skin
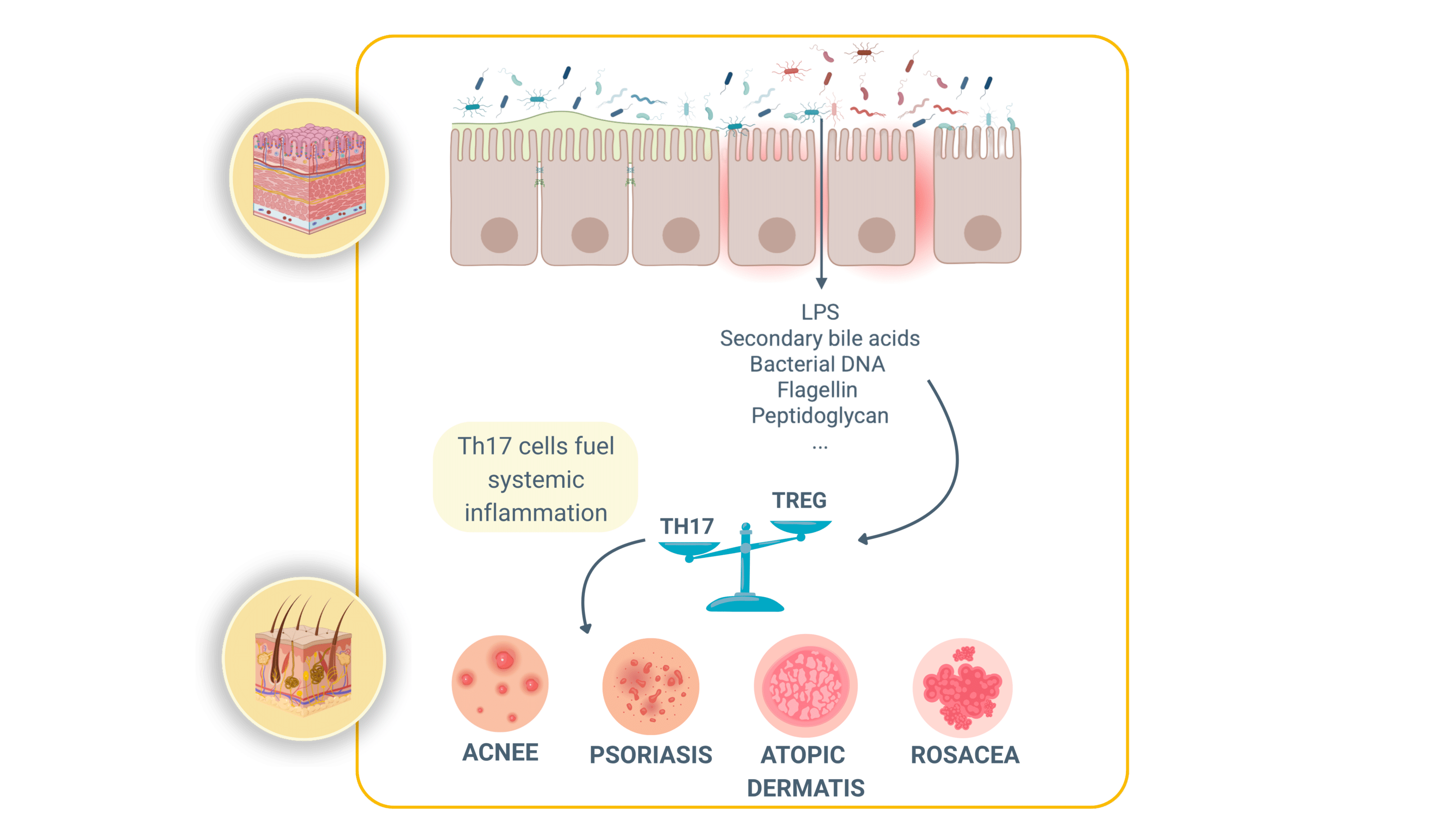
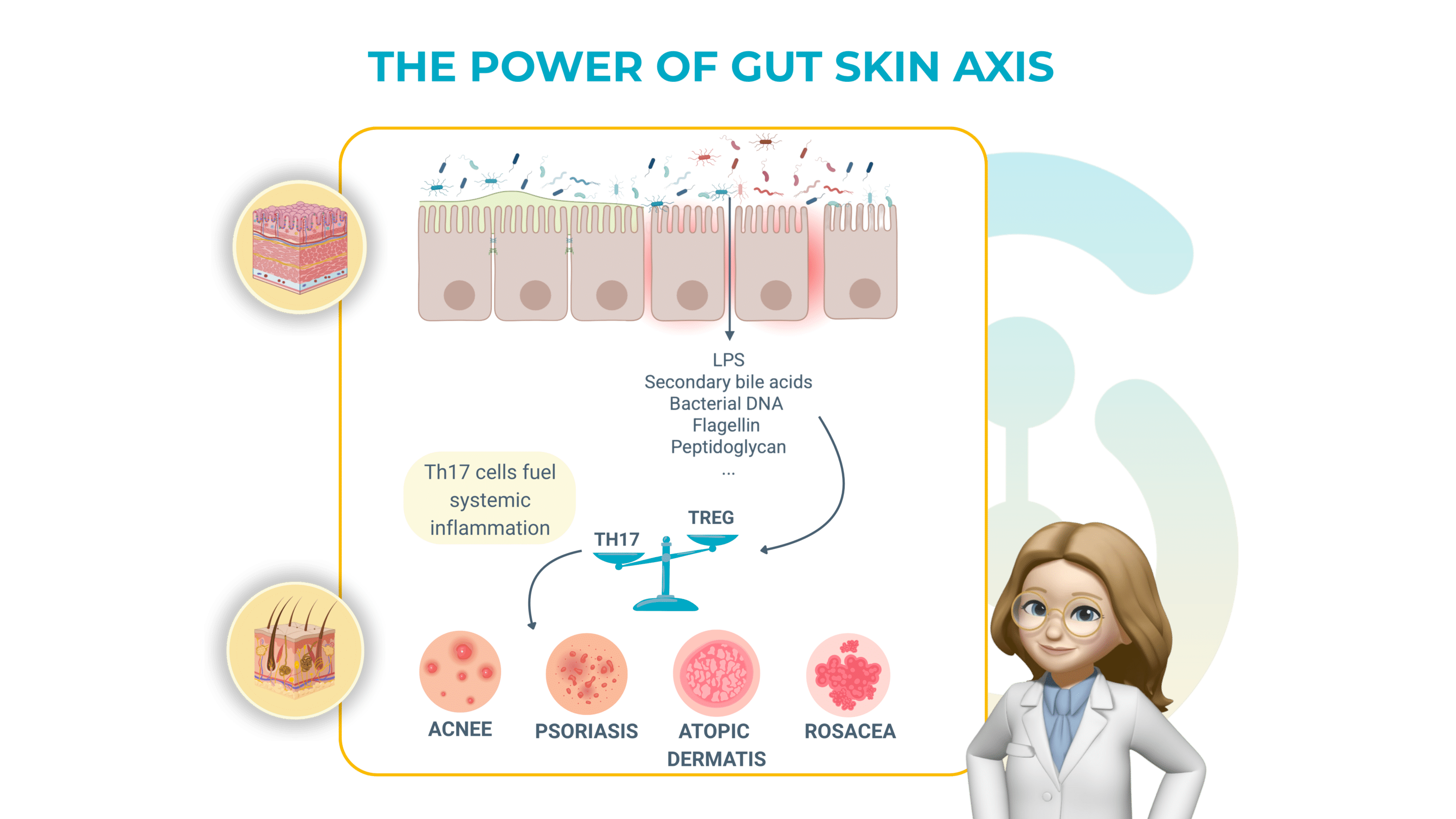
How a “leaky” gut inflames the skin:
The gut barrier function is triggered by intestinal cells that are zipped together by tight junctions. This barrier function keeps microbes and their fragments inside the gut. When those zippers loosen (because of different stress factors such as antibiotics, poor diet, etc.), three main troublemakers slip into the bloodstream:
> Lipopolysaccharide (LPS) : an alarm molecule from Gram-negative bacteria.
> Secondary bile acids: detergents that irritate immune cells outside the gut.
> Other pathogen-associated signals: bits of bacterial DNA, flagellin, peptidoglycan.
Once in the portal vein these signals hit the liver, our first immune checkpoint, and trigger a cascade of inflammatory messengers (notably TNF-α, IL-17, and IL-23). Those same cytokines are the drivers of psoriatic plaques, rosacea bumps and the painful sinus tracts of hidradenitis suppurativa (HS).
In short: a porous gut wall can broadcast a chemical “flare” that surfaces on the skin.
Why the conversation goes both ways
Sunlight (UV-B) on skin converts cholesterol into vitamin D₃. Activated vitamin D travels back to the intestine and switches on genes for antimicrobial peptides such as cathelicidin and defensins. These peptides :
> Kill over-grown microbes,
> Strengthen tight junction proteins,
> And ultimately reduce dysbiosis.
The skin therefore sends a “clean-up order” to the gut, proof that the axis is truly bidirectional.
Immune balancing act
Another example of crosstalk is the Th17/Treg ratio. Th17 cells fuel inflammation, while T regulatory (Treg) cells calm it. In both HS lesions and inflamed gut tissue this ratio skews toward Th17 dominance. Blocking TNF-α with adalimumab rebalances the ratio, improving both bowel and skin symptoms: a clinical reminder that treating one organ can reset immunity in the other!!
Next-gen in vitro models: Enterosys’ dual stage gut → skin platform
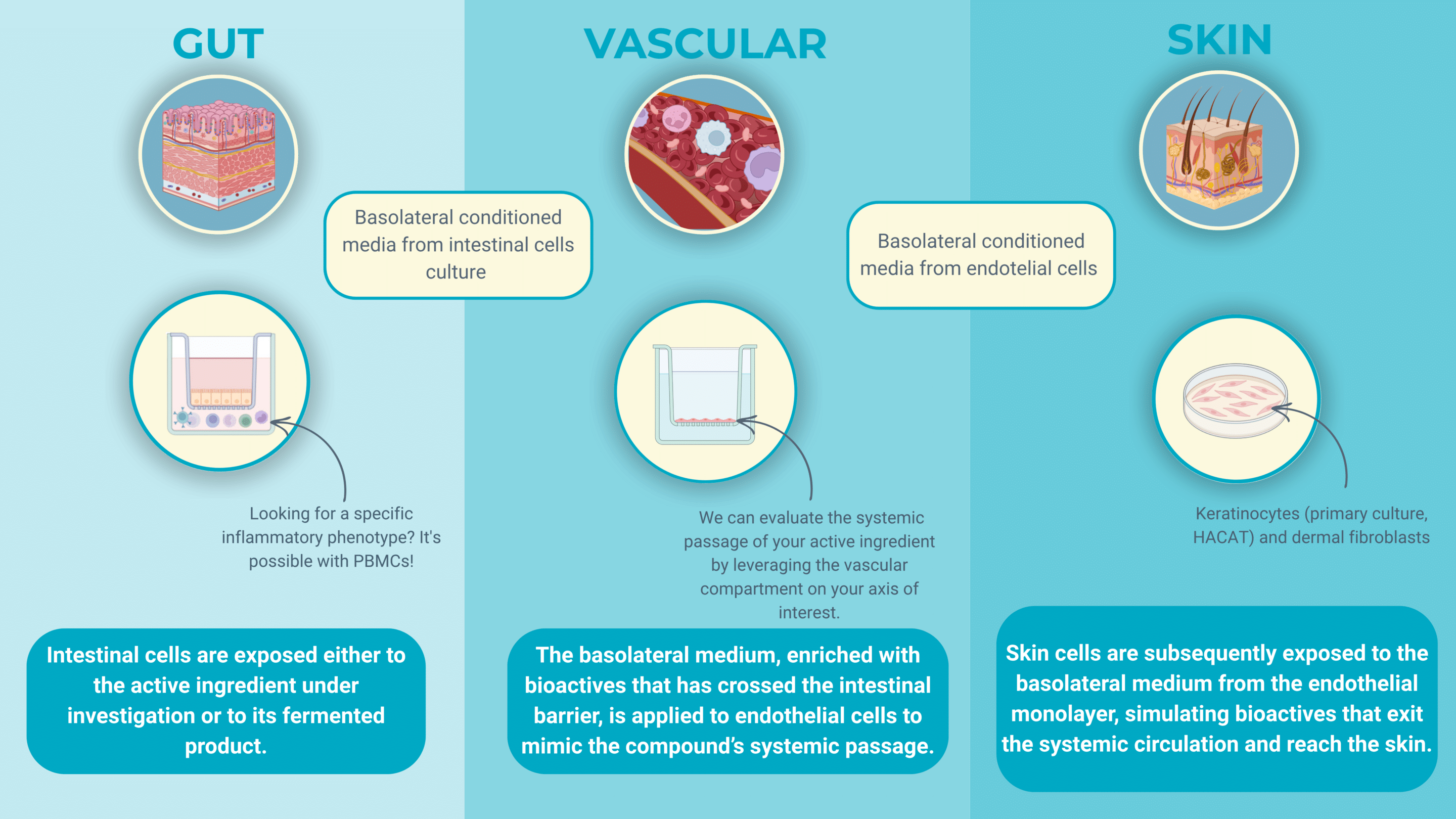
Enterosys has moved beyond simple co-cultures to a three-compartment system that recreates the entire gut–skin travel path of a nutricosmetic active. First, differentiated intestinal epithelial cells grow on a insert; immune sentinels sit in the basolateral well. Candidate ingredients are fermented to mimics colonic conditions, and the resulting supernatant is applied to the apical (luminal) side of the intestinal layer.
Real-time read-outs, short-chain-fatty-acid (SCFA) production, leakage and cytokine release (IL-8, TNF-α, IL-17), tell whether the compound tightens the gut barrier and how innate immunity reacts.
The conditioned basolateral medium is transferred to secondary cultures of human keratinocytes or dermal fibroblasts. Here the focus shifts to cutaneous endpoints: SASP expression (senescence & aging), ROS levels, cell differentiation and barrier function.
Because every nutricosmetic candidate passes through the full gut-fermentation, intestinal barrier, immune crosstalk and skin sequence, formulators can down-select only those actives that perform on both biological fronts, slashing time and cost compared with single-tissue assays and bringing truly gut-centric skin benefits to market faster.
Next-wave bioactives: from live consortia to precision postbiotics
A Paradigm shift in dermatological assessment
Curently new solutions are developed to nourish anti-inflammatory gut species and directly fortify the epidermis, offering a two-pronged approach that sidesteps the viability and tolerability issues of conventional probiotics :
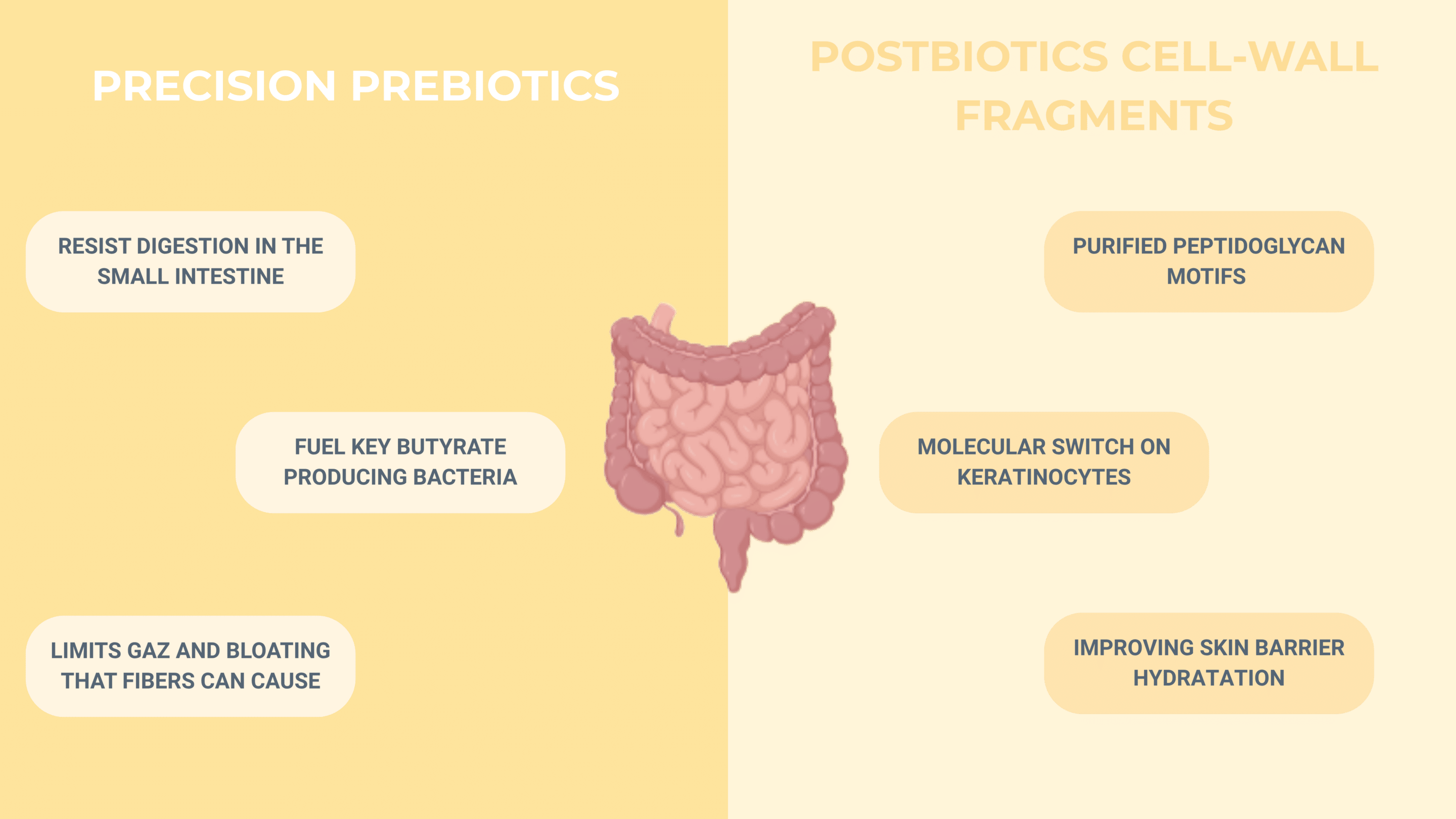
Precision prebiotics are specially engineered, low-FODMAP fibres produced under axenic conditions. Because they resist digestion in the small intestine yet are the preferred fuel of key butyrate-producing bacteria (Faecalibacterium prausnitzii, Roseburia spp.), they raise protective SCFA levels without triggering the gas and bloating that broad-spectrum fibres can cause.
Postbiotic cell-wall fragments take the concept one step further: instead of delivering live microbes, they supply purified peptidoglycan motifs, especially muramyl-dipeptide, that act as molecular “switches” on keratinocytes. These fragments bind NOD2 receptors and boost filaggrin and involucrin transcription, tightening the stratum corneum and improving barrier hydration even when no live bacteria are present.
Future studies will need to escape “skin-only” endpoints. Because the gut–skin axis is a crowded conversation, epithelial junctions, immune cells, microbial metabolites, endothelial barriers and cutaneous targets all speaking at once, no single read-out can do it justice. Pre-clinical platforms now let us model each relay in vitro, from colonic fermentation through intestinal uptake, endothelial transit and, finally, keratinocyte or fibroblast response.
In the end, the signal may fire from either direction, but the gut and the skin always answer each other. A leaky intestinal wall can shower the liver with LPS, secondary bile acids and other microbial alarms, lighting up TNF-α/IL-17 pathways that break out as plaques, papules or tunnels. Conversely, UV-B on the epidermis forges vitamin D₃, up-regulates cathelicidin in the gut and reins in dysbiosis. Enterosys’ triple-compartment model now tracks this journey step by step, while multi-strain synbiotics, precision prebiotics and postbiotic fragments show how we can calm both barriers at once.
So where does the cascade truly ignite, inside the lumen or on the surface? Share your verdict !
To support and structure this article, the following peer-reviewed scientific publications were used as primary sources of information:
- Egeberg, A., Hansen, P. R., Gislason, G. H., & Thyssen, J. P. (2017). Rosacea and gastrointestinal disorders: A Danish nationwide cohort study. Journal of the European Academy of Dermatology and Venereology, 31(5), 862–868. https://doi.org/10.1111/jdv.14004
- Garg, A., Papagermanos, V., Midura, M., Papadopoulos, D., & Jimenez, J. (2017). Hidradenitis suppurativa and inflammatory bowel disease: A systematic review and meta-analysis. Journal of Investigative Dermatology, 137(7), 1600–1608. https://doi.org/10.1016/j.jid.2017.02.980
- Jimenez-Sanchez, M., Celiberto, L. S., Yang, H., & Sham, H. P. (2023). Psoriasis, inflammatory bowel disease and colorectal cancer: A population-based meta-analysis of 7.8 million individuals. Frontiers in Microbiology, 14, 1192543. https://doi.org/10.3389/fmicb.2023.1192543
- Frew, J. W., Navrazhina, K., & Krueger, J. G. (2021). The Th17/Treg axis in hidradenitis suppurativa and gut inflammation: Immunological links and therapeutic implications. Frontiers in Immunology, 12, 720393. https://doi.org/10.3389/fimmu.2021.720393
- Parodi, A., Paolino, S., Greco, A., Gallo, M., Brembilla, A., & Drago, F. (2008). Small-intestinal bacterial overgrowth eradication with rifaximin markedly improves papulopustular rosacea. Clinical Gastroenterology and Hepatology, 6(7), 759–762. https://doi.org/10.1016/j.cgh.2008.03.007
- Huang, R., Atefi, N., Amin, K., et al. (2021). Multi-strain probiotic supplementation reduces SCORAD and restores skin-barrier proteins in adults with atopic dermatitis: A randomized, double-blind, placebo-controlled trial. European Journal of Dermatology, 31(2), 173–180. https://doi.org/10.1684/ejd.2021.4165
- Lee, K., Kim, H. J., Kim, S. A., Park, S.-D., Shim, J.-J., & Lee, J.-L. (2021). Exopolysaccharide from Lactobacillus plantarum HY7714 protects against skin ageing through gut–skin axis communication. Molecules, 26(6), 1651. https://doi.org/10.3390/molecules26061651
- Benyacoub, C., et al. (2014). Probiotics and skin health: A review of the evidence. Beneficial Microbes, 5(2), 143–151. https://doi.org/10.3920/BM2013.0040
- Searle, T., Ali, F. R., & Al-Niaimi, F. (2020). Rosacea and the gastrointestinal system: A systematic review. Australasian Journal of Dermatology, 61(3), 268–276. https://doi.org/10.1111/ajd.13198
- Maarouf, M., Cid, C., Garcia-Massague, A., et al. (2018). The role of nutrition in inflammatory pilosebaceous disorders: Implications of the gut–skin axis. Australasian Journal of Dermatology, 59(4), e198–e204. https://doi.org/10.1111/ajd.12799